Potential First-In-Class Pan-RAS Inhibitors Exhibit Efficacy in Variety of Difficult-To-Treat In Vivo Cancer Models
Qualigen is developing a platform of small molecule Pan-RAS oncogene protein-protein interaction inhibitors for treating adult and pediatric RAS-driven tumors with high unmet medical need. We are looking to leverage our compelling in vivo xenograft data to identify lead candidates for IND-enabling studies that address potential mechanisms of emerging KRAS G12C resistance through a pan-RAS approach.
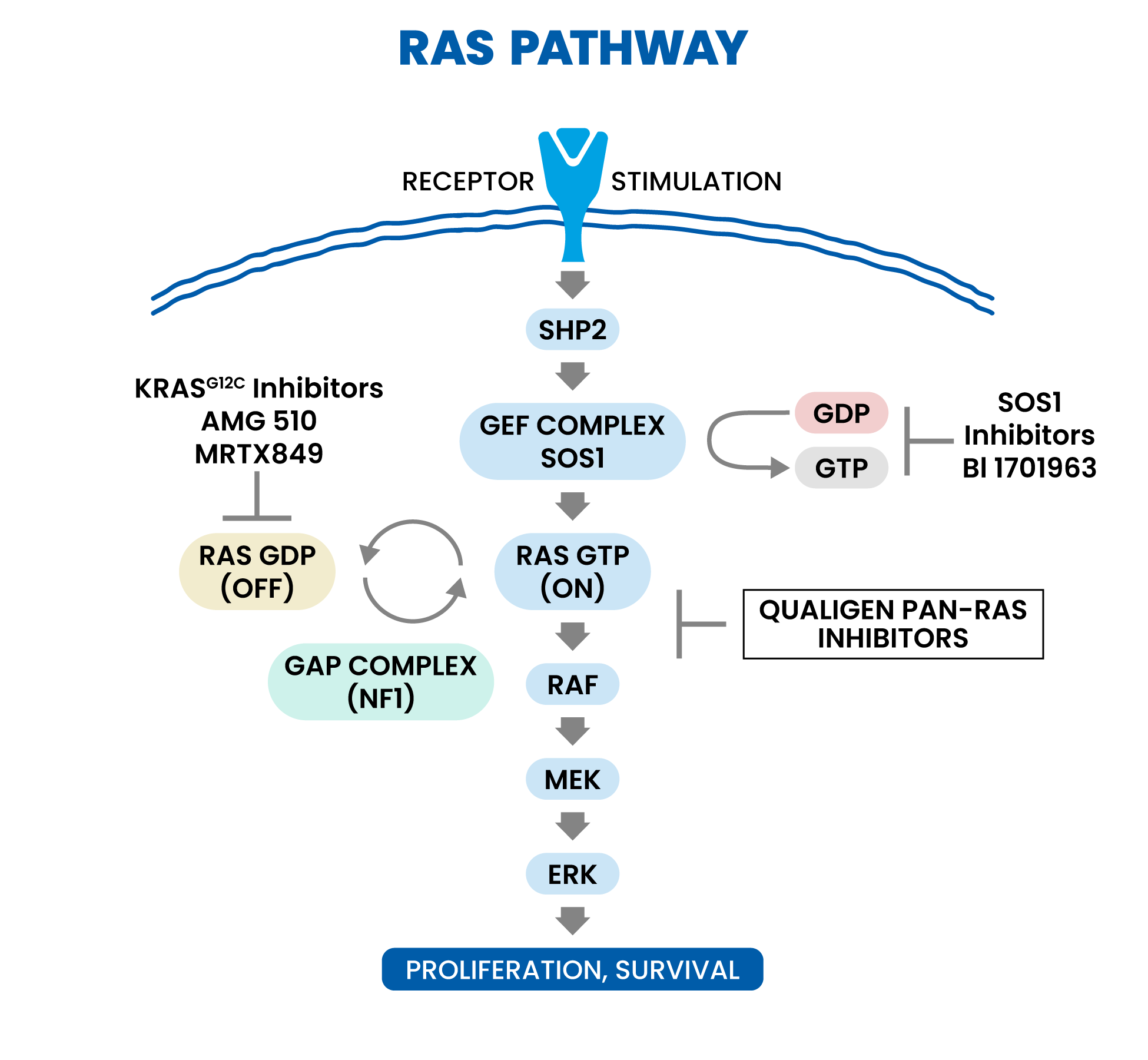
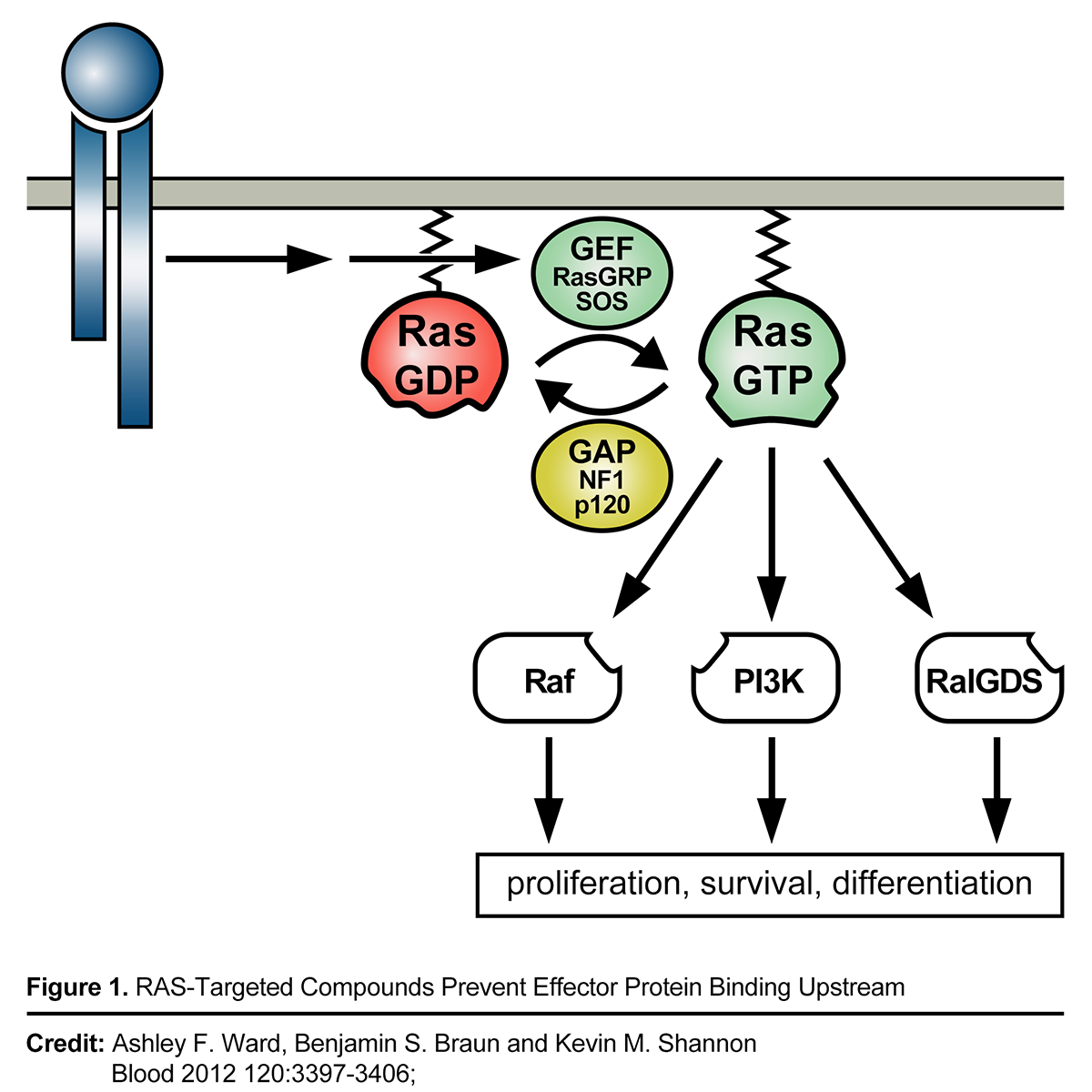
Mechanistic Rationale for pan-RAS Inhibition
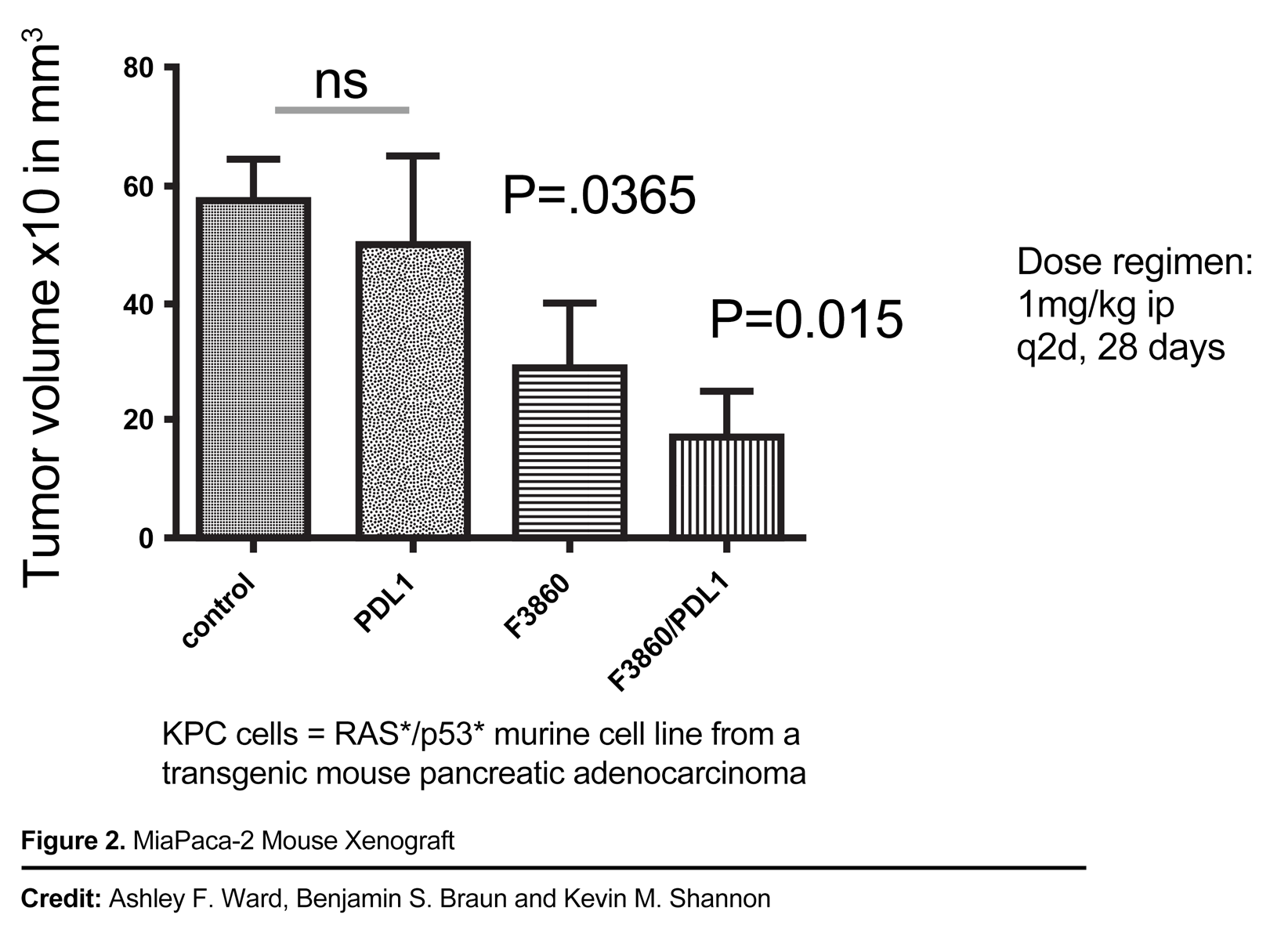
Pan-RAS Inhibitors as Treatments for Advanced Solid Tumors
RAS is the most common cancer oncogene, present in approximately a quarter or more of all cancers. Activating mutations in one of the three human RAS gene isoforms (KRAS, HRAS, or NRAS) are present in many tumor types, including 98% of pancreatic ductal adenocarcinomas, 52% of colon cancers, and 32% of lung adenocarcinomas. Cancers with mutant KRAS are diagnosed in more than 170,000 people each year in the US and cause more than 120,000 deaths for these three cancers alone. We believe novel mechanisms of action are desperately needed to address limitations in current standard of care such as chemotherapies like gemcitabine for treatment of these deadly cancers. In 2021, the FDA approved the first KRAS G12C targeted therapy, Sotorasib, under an accelerated approval for the treatment of KRAS G12C-mutated locally advanced or metastatic NSCLC (Non Small Cell Lung Cancer) in adult patients who have received at least one prior systemic therapy. However, emerging KRAS G12C resistance data affecting depth and duration of responses suggests a need for a pan-RAS approach that may address KRAS G12C resistance mechanisms.
Potential Advantages
- Inhibit KRAS G12C (MiaPaca-2) and KRAS G12D (Panc-1) at low micromolar concentrations
- Bind HRAS and NRAS in low micromolar concentrations
- Exhibit dose-sensitive effect on RAS/RAF complex in MiaPaca-2 cells
- Inhibit Wild-Type (WT) RAS-driven tumors in vivo
- Robust in-vivo anti-tumor activity in RAS-expressed mouse xenograft models
- Potential synergy with immune checkpoint therapy in vivo
- No serious adverse effects or weight differentiation with controls observed in mice at efficacious doses
- Composition of matter IP protection covering Qualigen’s RAS portfolio
- Potential to overcome lack of clinical durability with existing KRAS inhibitors by binding to RAS effector loop and overcoming resistance mechanisms associated with a single mutant isoform
Please contact [email protected] for more information.
Posters & Publications
- Poster – AACR Special Conference: Advances in Breast Cancer Research, October 2023
Pan-RAS Inhibitors to Treat Luminal B Breast Cancer. - Poster – 2023 ASCO Annual Meeting, June 2023
Impact of novel pan-RAS inhibitors on efficacy and resistance to AMG-510 and MRTX-1133 in pancreatic cancer cell lines. - Abstract – 2023 ASCO Annual Meeting, June 2023
A novel pan-RAS inhibitor for luminal B breast cancer. - Poster – AACR Special Conference: Targeting RAS, March 2023
A Novel RAS inhibitor for Luminal B and Triple Negative Breast Cancers - Poster – AACR Special Conference: Targeting RAS, March 2023
A Novel RAS inhibitor for Pancreatic Cancer - Poster – The Fourth RAS Initiative Symposium 2022
A novel pan-RAS inhibitor for Malignant Peripheral Nerve Sheath Tumors (Authors: Tariq Arshad, Geoff Clark) - Poster – The Fourth RAS Initiative Symposium 2022
A Novel RAS inhibitor for Pancreatic Cancer (Authors: Tariq Arshad, Geoff Clark) - Abstract – ASCO Annual Meeting, June 2022
A Novel RAS Inhibitor for Pancreatic Ductal Adenocarcinoma (Authors: Tariq Arshad, Geoff Clark) - Abstract – ASCO Annual Meeting, June 2022
A Novel Pan-RAS Inhibitor for Malignant Peripheral Nerve Sheath Tumors (Authors: Tariq Arshad, Geoff Clark) - Research Article – Molecular and Cellular Biology (journals.asm.org), December 2020
p75-Ras-GRF1 Is a c-Jun/AP-1 Target Protein: Its Up Regulation Results in Increased Ras Activity and Is Necessary for c-Jun-Induced Nonadherent Growth of Rat1a Cells - Research Article – Journal of Thoracic Disease (jtd.amegroups.com), May 2019
The role of RASSF proteins in modulating RAS driven lung tumors in vivo - Poster – Novel RAS inhibitors for NF1 disease – 2019
- Research Article – Cancer Research (cancerres.aacrjournals.org), May 2018
RASSF1A Deficiency Enhances RAS-Driven Lung Tumorigenesis - Research Article – BMC Cancer (bmccancer.biomedcentral.com), April 2018
Micro-RNA-186-5p inhibition attenuates proliferation, anchorage independent growth and invasion in metastatic prostate cancer cells - Research Article – Cancer Letters (sciencedirect.com), April 2017
The Role of the NORE1A Tumor Suppressor in Oncogene Induced Senescence - Research Article – Hepatology (aasldpubs.onlinelibrary.wiley.com), January 2017
NS5B Promotes Degradation of the NORE1A Tumor Suppressor to Facilitate Hepatitis C Virus Replication - Research Article – Seminars in Cell & Developmental Biology (sciencedirect.com), June 2016
Ras signaling through RASSF proteins - Research Article – Molecular & Cellular Oncology (tandfonline.com), April 2016
Nore1a drives Ras to flick the P53 senescence switch - Research Article – Cancers (mdpi.com), March 2016
NORE1A Regulates MDM2 Via β-TrCP - Research Article – Oncotarget (oncotarget.com), March 2016
Rheb may complex with RASSF1A to coordinate Hippo and TOR signaling - Research Article – Journal of Biological Chemistry (jbc.org), December 2015
Ras Regulates Rb via NORE1A - Research Article – Frontiers in Genetics (frontiersin.org), August 2015
A porcine model system of BRCA1 driven breast cancer - Research Article – Journal of Cell Biology (rupress.org), March 2015
NORE1A is a Ras senescence effector that controls the apoptotic/senescent balance of p53 via HIPK2